Calcium Atomic Number
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. Metallic calcium was first isolated by Sir Humphry Davy in 1808 through the electrolysis of a mixture of lime (CaO) and mercuric oxide (HgO). Today, metallic calcium is obtained by displacing calcium atoms in lime with atoms of aluminum in hot, low-pressure containers. About 4.2% of the earth's crust is composed of calcium.
Element Calcium (Ca), Group 2, Atomic Number 20, s-block, Mass 40.078. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of He 2s 2 2p 2, since its atomic number is 6. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of calcium-40 (atomic number: 20), the most common isotope of this element. The nucleus consists of 20 protons (red) and 20 neutrons (orange). 20 electrons (white) occupy available electron shells (rings).
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!
and our community of experts to gain a global audience for your work!  Timothy P. Hanusa
Timothy P. HanusaCalcium (Ca), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. It is the most abundant metallic element in the human body and the fifth most abundant element in Earth’s crust.
| atomic number | 20 |
|---|---|
| atomic weight | 40.078 |
| melting point | 842 °C (1,548 °F) |
| boiling point | 1,484 °C (2,703 °F) |
| specific gravity | 1.55 (20 °C, or 68 °F) |
| oxidation state | +2 |
| electron configuration | 1s22s22p63s23p64s2 |

Occurrence, properties, and uses
Calcium does not occur naturally in the free state, but compounds of the element are widely distributed. One calcium compound, lime (calcium oxide, CaO) was extensively used by the ancients. The silvery, rather soft, lightweight metal itself was first isolated (1808) by Sir Humphry Davy after distilling mercury from an amalgam formed by electrolyzing a mixture of lime and mercuric oxide. The name for the element was taken from the Latin word for lime, calx.
Calcium constitutes 3.64 percent of Earth’s crust and 8 percent of the Moon’s crust, and its cosmic abundance is estimated at 4.9 × 104atoms (on a scale where the abundance of silicon is 106 atoms). As calcite (calcium carbonate), it occurs on Earth in limestone, chalk, marble, dolomite, eggshells, pearls, coral, stalactites, stalagmites, and the shells of many marine animals. Calcium carbonate deposits dissolve in water that contains carbon dioxide to form calcium bicarbonate, Ca(HCO3)2. This process frequently results in the formation of caves and may reverse to deposit limestone as stalactites and stalagmites. As calcium hydroxyl phosphate, it is the principal inorganic constituent of teeth and bones and occurs as the mineralapatite. As calcium fluoride, it occurs as fluorite, or fluorspar. And as calcium sulfate, it occurs as anhydrite. Calcium is found in many other minerals, such as aragonite (a type of calcium carbonate) and gypsum (another form of calcium sulfate), and in many feldspars and zeolites. It is also found in a large number of silicates and aluminosilicates, in salt deposits, and in natural waters, including the sea.
Formerly produced by electrolysis of anhydrous calcium chloride, pure calcium metal is now made commercially by heating lime with aluminum. The metal reacts slowly with oxygen, water vapour, and nitrogen of the air to form a yellow coating of the oxide, hydroxide, and nitride. It burns in air or pure oxygen to form the oxide and reacts rapidly with warm water (and more slowly with cold water) to produce hydrogen gas and calcium hydroxide. On heating, calcium reacts with hydrogen, halogens, boron, sulfur, carbon, and phosphorus. Although it compares favourably with sodium as a reducing agent, calcium is more expensive and less reactive than the latter. In many deoxidizing, reducing, and degasifying applications, however, calcium is preferred because of its lower volatility and is used to prepare chromium, thorium, uranium, zirconium, and other metals from their oxides.
The metal itself is used as an alloying agent for aluminum, copper, lead, magnesium, and other base metals; as a deoxidizer for certain high-temperature alloys; and as a getter in electron tubes. Small percentages of calcium are used in many alloys for special purposes. Alloyed with lead (0.04 percent calcium), for example, it is employed as sheaths for telephone cables and as grids for storage batteries of the stationary type. When added to magnesium-based alloys in amounts from 0.4 to 1 percent, it improves the resistance of degradable orthopedic implants to biological fluids, permitting tissues to heal fully before the implants lose their structural integrity.
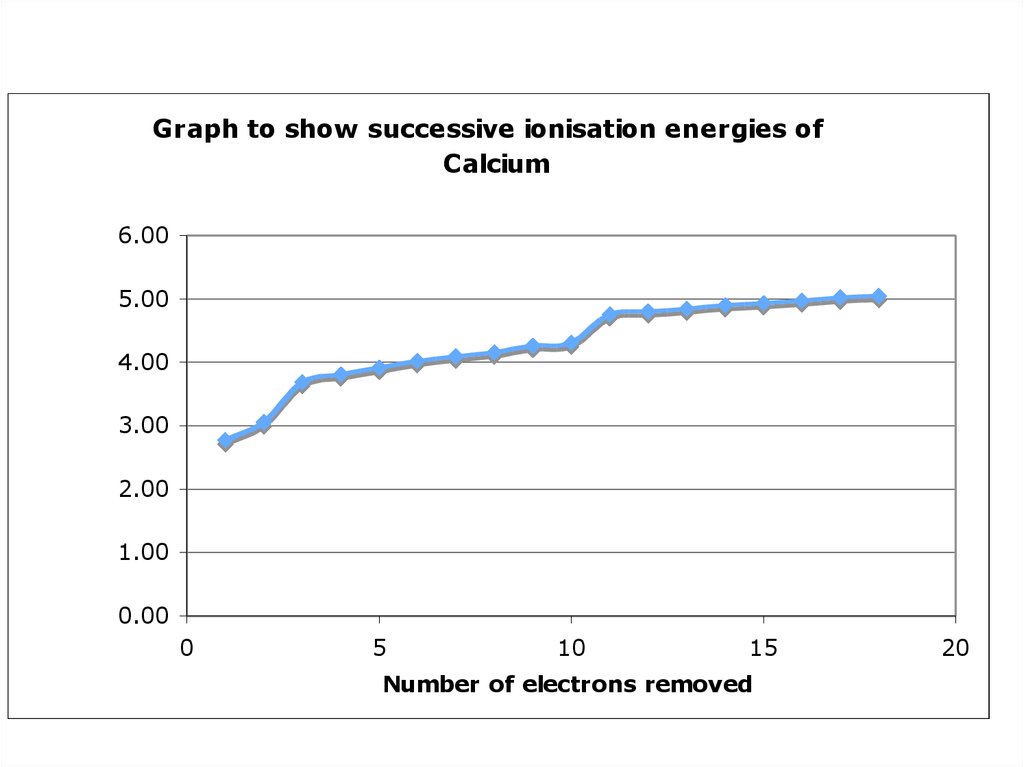
Naturally occurring calcium consists of a mixture of six isotopes: calcium-40 (96.94 percent), calcium-44 (2.09 percent), calcium-42 (0.65 percent), and, in smaller proportions, calcium-48, calcium-43, and calcium-46. Calcium-48 undergoes double beta decay with a half-life of roughly 4 × 1019 years, so it is stable for all practical purposes. It is particularly neutron-rich and is used in the synthesis of new heavy nuclei in particle accelerators. The radioactive isotope calcium-41 occurs in trace quantities on Earth through the natural bombardment of calcium-40 by neutrons in cosmic rays.
Calcium is essential to both plant and animallife and is broadly employed as a signal transducer, enzymecofactor, and structural element (e.g., cell membranes, bones, and teeth). A large number of living organisms concentrate calcium in their shells or skeletons, and in higher animals calcium is the most abundant inorganic element. Many important carbonate and phosphate deposits owe their origin to living organisms.
The human body is 2 percent calcium. Major sources of calcium in the human diet are milk, milk products, fish, and green leafy vegetables. The bone diseaserickets occurs when a lack of vitamin D impairs the absorption of calcium from the gastrointestinal tract into the extracellular fluids. The disease especially affects infants and children.
- key people
- related topics
Atomic Number of Calcium is 20.
Chemical symbol for Calcium is Ca. Number of protons in Calcium is 20. Atomic weight of Calcium is 40.078 u or g/mol. Melting point of Calcium is 839 °C and its the boiling point is 1487 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearAbout Calcium
One of the most important elements for humans and animals, calcium is a light and soft metal of light grey color. It is very reactive, especially with water and air. It has got its name from a Latin word meaning lime. It is impossible to find pure calcium in nature, but it can be found in abundance in various chemical compounds, thus it is considered to be the fifth most abundant element on the surface of earth. We receive calcium with foods and when brushing our teeth. In a human body, we have about 1 kilogram of calcium which is mainly in our bones and teeth. Calcium is used in metallurgy to produce alloys with other metals, as well as in chemical industry or for producing various goods like paper, food, products from clay, and others. Calcium and especially a few of its compounds are very toxic.
Uses of Calcium
Calcium, a silvery-white, alkaline earth metal with the symbol Ca, is mostly used in steelmaking. Calcium compounds are commonly used in foods, paints, toothpaste, rubbers, papermaking, baking, and medicine. They are used as pharmaceuticals as well as for laboratory purposes too. Calcium carbonate is mainly used in the construction industry. It is also preferred in the oil industry, and it can be added to swimming pools.
Calcium metal is used as an alloying agent for aluminum, copper, and magnesium. Tricalcium phosphate (abbreviated TCP) can be found in baby powders and toothpaste. Calcium fluoride, a compound with the elements calcium and fluorine, is used in the manufacture optical components like lenses, windows as well as telescopes, spectroscopy, etc. Calcium pyrophosphate, another compound with the formula Ca2P2O7, is widely used in toothpaste as a mild abrasive agent.
Compounds with Calcium
- CaCO3: Calcium carbonate
- CaC2: Calcium carbide
- CaF2: Calcium fluoride
- Ca(OH)2: Calcium hydroxide
- CaCl2: Calcium chloride
- CaCN2: Calcium cyanamide
- CaO: Calcium oxide
- CaSO4: Calcium sulfate
- Ca2P2O7: Calcium pyrophosphate
- Ca3(PO4)2: Tricalcium phosphate
- C6H10CaO6: Calcium lactate
- CaSO3: Calcium sulfite
- Ca2O4Si: Calcium silicate
- C4H6O4Ca: Calcium acetate
Properties of Calcium Element
| Atomic Number (Z) | 20 |
|---|---|
| Atomic Symbol | Ca |
| Group | 2 |
| Period | 4 |
| Atomic Weight | 40.078 u |
| Density | 1.54 g/cm3 |
| Melting Point (K) | 1115 K |
| Melting Point (℃) | 839 °C |
| Boiling Point (K) | 1757 K |
| Boiling Point (℃) | 1487 °C |
| Heat Capacity | 0.647 J/g · K |
| Abundance | 41500 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Alkaline earth metal |
| Electronegativity (Pauling) χ | 1 |
| Ionization Energy (eV) | 6.11316 |
| Atomic Radius | 180pm |
| Covalent Radius | 174pm |
| Valence Electrons | 2 |
| Year of Discovery | 1808 |
| Discoverer | Davy |
What is the Boiling Point of Calcium?
Calcium boiling point is 1487 °C. Boiling point of Calcium in Kelvin is 1757 K.
What is the Melting Point of Calcium?
Calcium Atomic Number 20
Calcium melting point is 839 °C. Melting point of Calcium in Kelvin is 1115 K.
How Abundant is Calcium?
Abundant value of Calcium is 41500 mg/kg.
What is the State of Calcium at Standard Temperature and Pressure (STP)?
State of Calcium is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Calcium Discovered?
Calcium was discovered in 1808.
Calcium Atomic Number Bohr Model
